History, Status, Impacts, and Management of Invasive Black Spiny-tailed Iguanas (Ctenosaura similis) on Gasparilla Island, Florida
This publication summarizes the history, status, and management of invasive black spiny-tailed iguanas (Ctenosaura similis) on Gasparilla Island, in southwest Florida. Our target audiences are residents and visitors of Gasparilla Island as well as naturalists interested in Florida’s diverse wildlife, especially introduced reptiles. Our specific goal is to increase knowledge and raise awareness about black spiny-tailed iguanas, their impacts, and the challenges of managing those impacts. More broadly, we aim to raise awareness about the many introduced and invasive reptiles in Florida and motivate people to take action to help prevent additional introductions of nonnative reptiles as well as support management efforts to curtail the spread of introduced reptiles in the Sunshine State. The information used to produce this publication was derived from published scientific articles, communications with colleagues, and our own research.
Invasive Species
A species is considered invasive when it is introduced by humans to an area where it is not native, and it causes or could cause damage to the environment, economy, or human health and safety in that area (Iannone et al. 2021). Invasive species are a major threat to global biodiversity, causing the extinction of at least 34 species and contributing to the extinction of at least 57 others (Clavero and García-Berthou 2005). Invasive species also cause about $120 billion in damages and associated management costs per year in the United States alone (Pimental et al. 2005).
Florida has more established nonnative reptile and amphibian species than anywhere else in the world. Over 60 species of introduced reptiles and amphibians have established breeding populations within the state (Krysko et al. 2016). In fact, Florida has three times as many established species of introduced lizards as native lizards (Hardin 2007). Florida is a prime spot for invasion by nonnative reptiles due to several factors. Florida is a shipping hub with major ports of entry for arrival of wildlife species (both legal and illegal) (Lockwood et al. 2019). These species may be the commodity being shipped, as in the pet trade, or transported inadvertently as “stowaways” in cargo (King and Krakauer 1966). The state has a popular captive wildlife industry, and facilities housing nonnative species (e.g., breeders, retail vendors, individual pet owners) are susceptible to escapes and sometimes intentional releases of unwanted animals (Fujisaki et al. 2010) (Episcopio-Sturgeon and Pienaar 2019). Additionally, Florida’s mild climate is suitable for many introduced amphibians and reptiles (Bomford et al. 2008) (Engeman et al. 2011). Much of Florida has a sub-tropical climate, and although the entire state lies north of the Tropic of Cancer, the very southern portion of the Peninsula is considered tropical. These conditions resemble the climate in the native ranges of many of the nonnative reptiles that are now established in Florida.
The Burmese python is probably the best known of Florida’s invasive reptiles, frequently making headlines because of the snake’s massive size and occasional interactions with alligators. Introduced through the pet trade, pythons were first recorded in the Everglades in 1979 and have been considered established there since 2000 (Guzy et al. 2023). Studies have shown that pythons have caused dramatic declines in mammal populations in the Everglades (Dorcas et al. 2012) (McCleery et al. 2015), and they have spread invasive parasites, which are affecting native snakes (Miller et al. 2018). They may also have a considerable impact on native bird populations (Dove et al. 2011). Government agencies have spent millions of dollars on research and management in an attempt to prevent further spread of this invasive snake in Florida (Guzy et al. 2023). While they are the most infamous, Burmese pythons are but one of many invasive reptiles harming Florida’s economy, environment, and human quality of life.
History of Black Spiny-tailed Iguanas on Gasparilla Island
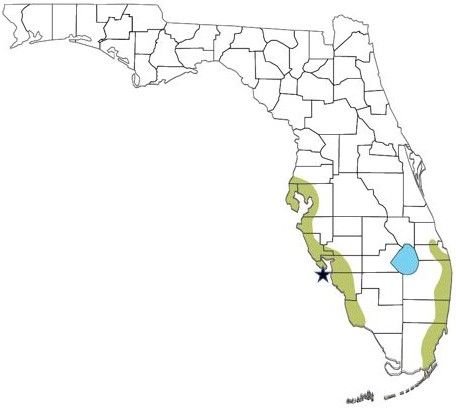
Black spiny-tailed iguanas (Ctenosaura similis) occur naturally from Mexico to Panama (Fitch and Hackforth-Jones 1983). They were introduced to Gasparilla Island intentionally by a resident who apparently brought back three iguanas as pets from Mexico between the late 1970s and the early 1980s. He and his children raised the iguanas for several years and then released them near the Range (aka Gasparilla Island) Lighthouse on the southern end of the island (Krysko et al. 2003). The iguanas apparently were not hindered by inbreeding due to their small starting population size. They remained localized on the south side of the island for a while, but by 2003 they had spread across the entire island and onto the mainland (Krysko et al. 2003). Due to numerous reasons discussed below, black spiny-tailed iguanas are considered an invasive species in Florida.
Today, black spiny-tailed iguanas occur along much of the southern half of Florida’s Gulf Coast (including Gasparilla Island) as well as the south Atlantic Coast (Figure 1). Analysis of DNA suggests the majority, if not all, of the black spiny-tailed iguanas on the west coast are derived from the initial introduction and subsequent spread of the species from the three pet iguanas on Gasparilla Island (Nunez 2016). The black spiny-tailed iguanas on the east coast appear to be from several additional and independent introductions of iguanas associated with the pet trade (Nunez 2016). There is also an isolated population of Mexican spiny-tailed iguanas in a small area near the Charles Deering Estate in Miami.
Credit: Tracy Bryant, UF/IFAS
Identification and Ecology of Black Spiny-tailed Iguanas
Black spiny-tailed iguanas are the only large nonnative lizard on Gasparilla Island, making identification there straightforward. Hatchlings are around six inches long, including their tail, when they emerge from the egg. Sometimes they may be light green, but more typically they are pale tan to gray with dark brown netlike markings called “reticulations” (Figure 2) (Fitch and Hackforth-Jones 1983). Tan/gray hatchlings become bright green with brown, black, and gold markings within a few months of hatching (Lee 2000). Juveniles may or may not have dark crossbands on their back (Figure 3). Juveniles move quickly and may be seen running along the ground or climbing low trees or shrubs. After about six months, they attain their adult coloration of light gray or tan with dark bands across the back. Adults may reach lengths of greater than four feet, including their long, spiny tail. Males are larger than females and have stockier heads and a more pronounced crest of scales along their back (Figure 4). Their heads are usually light gray or tan, but during the breeding season, males will often develop orange coloring on the head with reddish-orange spots on the body. They have long tails with rings of prominent, spiny scales as their name suggests. The spines may be used for defense if the animals are picked up or attacked by a predator (Figure 5) (Fitch and Hackforth-Jones 1983) (Lee 2000). Other lizard species found on Gasparilla Island include the nonnative brown anole (Anolis sagrei) (more about brown anoles in Florida here) and tropical house gecko (Hemidactylus mabouia); as well as the native green anole (Anolis carolinensis) and six-lined racerunner (Aspidoscelis sexlineata). However, none of these grows longer than about 12 inches, including the tail, so they are not likely to be confused with black spiny-tailed iguanas. Several other large invasive lizards also occur in Florida, including the Nile monitor (Varanus niloticus), green iguana (Iguana iguana) (more about green iguanas in Florida here); and Argentine black and white tegu (Salvator marinae) (more about tegu lizards in Florida here); but none presently occur on Gasparilla Island.

Credit: Kevin Enge, FWC, used with permission

Credit: Steve A. Johnson, UF/IFAS

Credit: Steve A. Johnson, UF/IFAS

Credit: Sean L. McKnight, UF/IFAS
Juvenile black spiny-tailed iguanas feed almost exclusively on insects and other small invertebrates. They begin to consume more plant material as they get older but will still prey on animals when given the chance, including small mammals, birds and bird eggs, hatchling sea turtles, young gopher tortoises, and other lizards, including their own species (Avery et al. 2009) (Krysko et al. 2009) (Garcia Rosales et al. 2020).
Female black spiny-tailed iguanas become reproductive at two years of age. On Gasparilla Island, females lay a single group of eggs (called a clutch) each year in early summer, and hatchlings emerge in late summer. The number of eggs per clutch increases with the body size of the female and averages about 30 on the island, though up to 82 eggs have been found within a female in Florida (Krysko et al. 2003) (Avery et al. 2014). In their native range, females are known to lay their eggs in burrows in sunny, sandy areas. These nest burrows can be extensive, with numerous side tunnels, and up to five females may lay eggs in separate chambers within the burrow (Fitch and Hackforth-Jones 1983). However, their nesting behavior on Gasparilla Island is poorly documented.
Black Spiny-tailed Iguanas as an Invasive Species on Gasparilla Island
Black spiny-tailed iguanas harm people on Gasparilla Island in several ways. The iguanas may compromise the integrity of manmade structures by burrowing beneath the foundations of buildings, into rock walls and sea walls, under sidewalks, etc. (Figure 6). They damage landscaping plants by eating the flowers and leaves of ornamental vegetation and stripping bushes of foliage, as seen in this video of one of them climbing a hibiscus bush to eat a flower: https://original-ufdc.uflib.ufl.edu/IR00011963/00001/video.

Credit: Sean L. McKnight, UF/IFAS
When black spiny-tailed iguanas occur at high densities, their feces can become smelly, unsightly, and unsanitary, particularly in or around swimming pools and docks (Figure 7). Although there is no evidence that wild iguanas in Florida transmit pathogens to humans, their feces may contain bacteria such as E. coli and Salmonella (Sylvester et al. 2014) (Charruau et al. 2020). They have been known to nest in attics and climb through vents, and sometimes end up in people’s toilets (Skoloff 2006). Black spiny-tailed iguanas are not normally dangerous to people or pets. They are not aggressive and usually flee if approached. However, they are defensive and may inflict a painful bite and scratches with their claws and spiny tail if grabbed or cornered by a person or dog.

Credit: Eric Tillman, U.S. Department of Agriculture and previously published in a scientific journal, used with permission
Black spiny-tailed iguanas could harm sensitive native wildlife species on the island such as the gopher tortoise (Gopherus polyphemus), loggerhead sea turtle (Caretta caretta), green sea turtle (Chelonia mydas), least tern (Sterna antillarum), a seabird, and snowy plover (Chardrius nivosus), a shorebird. Predation on juvenile tortoises has been documented on the island (Avery et al. 2009) (Figure 7). In Mexico, they are known to eat the eggs and hatchlings of least terns and prey on hatchling sea turtles (Garcia Rosales et al. 2020). The extent to which iguana predation affects the populations of any of these species in Florida is unknown.

Credit: Sean L. McKnight, UF/IFAS
Ecology and Importance of Gopher Tortoises
Gopher tortoises are native to the Southeastern Coastal Plain of the United States from Louisiana east to South Carolina and south to Florida. They require well-drained, sandy soil for digging burrows and nests, a sparse overstory with sunny places for basking and nesting, and plenty of short, tender plants to eat (Hipes et al. 2000). This terrestrial turtle is common on Gasparilla Island, especially among the sand dunes. They are brown to gray land-dwelling turtles that average 8 to 10 inches long (shell length) as adults but can reach lengths of 12 inches (Ernst and Lovich 2009) (Figure 9). Hatchlings are about two inches long (Figure 10). Gopher tortoises are slow to reproduce and have low survival when they are small. Females take 9–21 years to reach sexual maturity (Landers et al. 1982) (Godley 1989) (Mushinsky and McCoy 1994). Predators destroy the eggs of many tortoise nests, and most young are consumed by predators or die from other causes before they reach adulthood. Studies have found that over 90 percent of eggs and hatchlings are eaten before reaching one year old (Alford 1980) (Witz et al. 1991).

Credit: Sean L. McKnight, UF/IFAS

Credit: Steve A. Johnson, UF/IFAS
Gopher tortoises use their broad front feet and strong claws to dig long burrows for protection from predators, fire, dehydration, and extreme hot and cold temperatures (Hipes et al. 2000). Their burrows are usually easily recognizable (Figure 11). The opening is half-moon shaped and there is typically a mound of sand called an “apron” just outside the entrance, which is formed as the tortoise kicks out soil while digging the burrow. They spend most of their time in these burrows, leaving primarily to eat and bask in the sun (Douglass and Layne 1978). Gopher tortoises are considered ecosystem engineers because their burrows act as shelter, feeding, and reproduction sites for at least 360 other species, often referred to as “commensals,” some of which cannot exist without the tortoise (Jackson and Milstrey 1989). Gopher tortoises are also considered a keystone species because their presence promotes higher diversity of other species (Catano and Stout 2015). Click here for more information on gopher tortoises.

Credit: Sean L. McKnight, UF/IFAS
Iguana Use of Gopher Tortoise Burrows
Gopher tortoises are common on Gasparilla Island and are found within beach dune and coastal strand habitats, as well as in grassy areas near buildings and roads. Although iguanas often dig their own burrows, particularly under objects such as rocks and logs, they will also occupy the burrows of gopher tortoises, as in Figure 12, below, and in this video showing a black spiny-tailed iguana (Ctenosaura similis) entering a gopher tortoise burrow: https://original-ufdc.uflib.ufl.edu/IR00011950/00004.
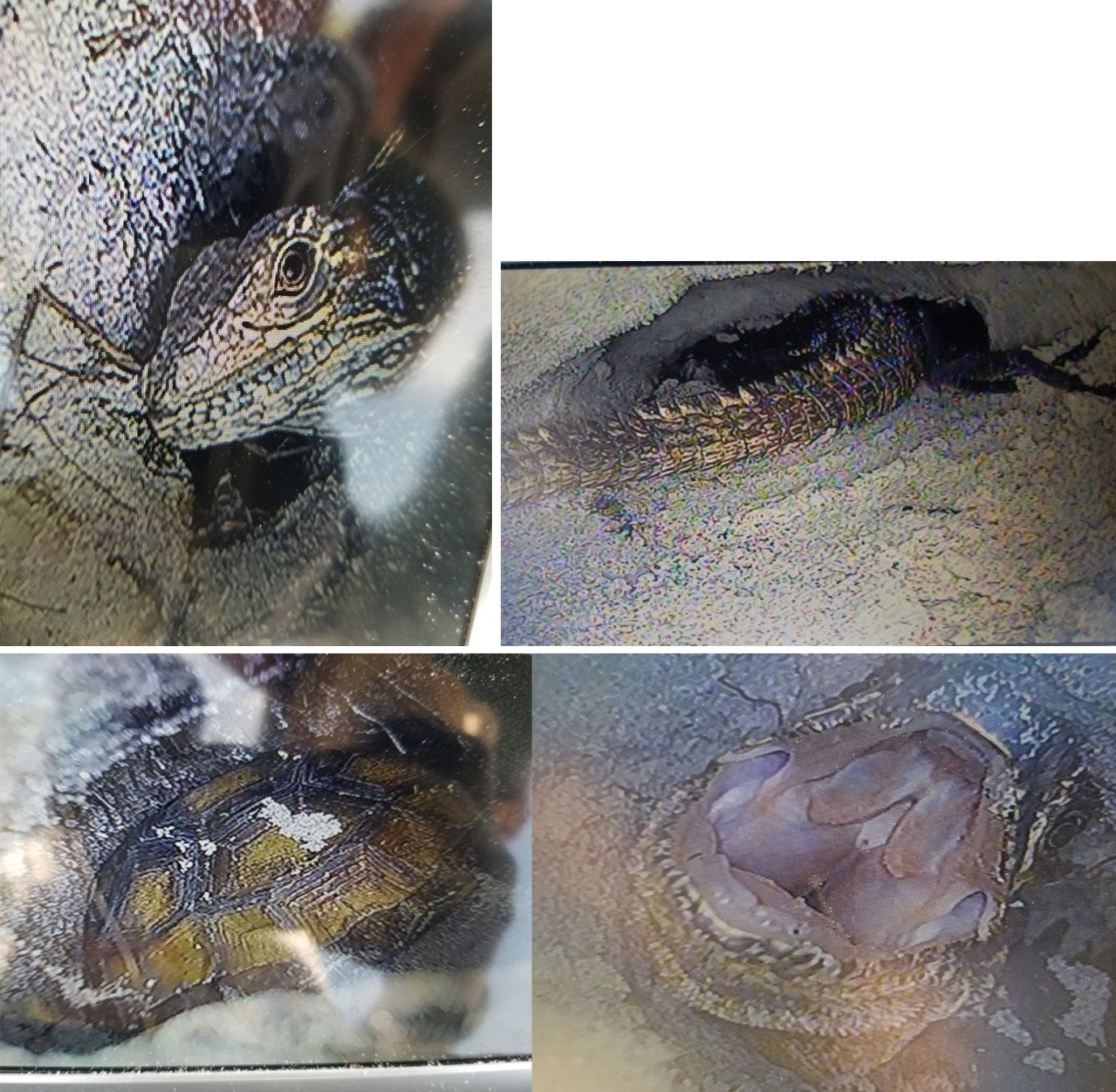
Credit: Sean L. McKnight, UF/IFAS
Research conducted by the lead author (S.L. McKnight) evaluated the interactions between black spiny-tailed iguanas and gopher tortoises on Gasparilla Island to see if iguanas moving into tortoise burrows changes tortoises’ burrowing behavior, and, if so, whether the changes may eventually harm gopher tortoises. Specifically, it has been suspected that iguanas may exclude tortoises from existing burrows and cause them to dig additional burrows (Engeman et al. 2009). This work took place at two sites within the beach dune habitat along the west edge of the island (Figure 13) where tortoise burrows are easy to find. One site was within Gasparilla Island State Park, which has had minimal iguana removal effort, and the other was within private property where there has been ongoing iguana removal by the U.S. Department of Agriculture Wildlife Services since 2008. A camera scope was used to look inside tortoise burrows (Figure 14), as well as side tunnels dug by iguanas into the end, sides, and ceilings of the tortoise burrows (Figure 15). Scoping footage showing iguana side-tunnels in a gopher tortoise burrow is available here: https://original-ufdc.uflib.ufl.edu/IR00011950/00003. Note the nonnative greenhouse frog in the lower tunnel. Scoping frequently revealed these frogs in gopher tortoise burrows.

Credit: Sean L. McKnight, UF/IFAS

Credit: Sean L. McKnight, UF/IFAS

Credit: Sean L. McKnight, UF/IFAS
Tortoise burrows were inspected in the morning during winter, while iguanas and tortoises would still be inside the burrows—both species are active during the day but seek shelter at night. Rarely (only 1.25% of burrows examined) were tortoises and iguanas observed in the same burrow at the same time, and tortoises were less likely to occupy burrows that contained side tunnels dug by iguanas. And on only a single occasion was more than one iguana observed in a burrow. However, at very high densities, the frequency of simultaneous use of burrows by multiple iguanas may increase. Tortoise occupancy was lower and iguana occupancy was higher in tortoise burrows at Gasparilla Island State Park, where there has been relatively little iguana removal compared to the private property site. Furthermore, tortoises were less likely to be seen in a burrow where iguanas were observed during previous surveys. Our research findings from Gasparilla Island suggest that tortoises are avoiding or being excluded from their burrows by the iguanas, causing tortoises to move and dig additional burrows. Although the precise mechanism is still unknown (e.g., tortoises leaving after a failed predation attempt or aggressive interaction, or avoiding iguana scent), Gasparilla Island State Park staff reported an observation of a brief “stand-off” between a very large iguana and a large tortoise at the park (Figure 16), where a tortoise blocked its burrow entrance and the iguana soon left. During the following days, both species were seen coming and going from the burrow, but eventually only the iguana was seen using the burrow.

Credit: Gasparilla Island State Park staff, used with permission
Additionally, a higher proportion of smaller tortoise burrows (dug by juvenile tortoises) was found at the state park, although the tortoise occupancy rate was dramatically lower there. At first glance, an abundance of burrows, especially juvenile burrows, may seem like a good thing—a sign of a healthy breeding population. However, a low tortoise-to-burrow ratio (or more burrows per tortoise) is considered a sign of environmental stress, as each tortoise uses more burrows when food is scarcer or disturbances by potential predators are more common (Mushinsky et al. 1994) (Wilson et al. 1994). We suggest that the higher proportion of juvenile burrows at the state park is likely the result of juvenile tortoises being disproportionately affected because they are potential prey for iguanas. The concerns over tortoises having to dig additional burrows include the energetic cost of excavating the burrow as well as the lost opportunity to forage while they are digging. In addition, tortoises are more exposed to predators, extreme temperatures, and dehydration while they search for a new location to burrow and when digging the burrow.
Other species observed inside tortoise burrows on Gasparilla Island included the native eastern coachwhip snake (Masticophis flagellum flagellum), southern ringneck snake (Diadophis punctatus punctatus), and southern toad (Anaxyrus terrestris), as well as an invasive Burmese python (Python bivittatus) and nonnative greenhouse frogs (Eleutherodactylus planirostrus) (see Figure 17 and scoping footage).

Credit: Sean L. McKnight, UF/IFAS
Managing Iguanas on Gasparilla Island
The residents of Gasparilla Island found the iguanas such a nuisance that they self-imposed a tax to pay for iguana management. Gasparilla Island is part of two counties, with the northern portion in Charlotte County and the southern portion in Lee County. In 2007, Lee County hired a private iguana trapper, and, in 2008, Charlotte County contracted USDA Wildlife Services to remove iguanas (Avery et al. 2014). The trappers have access to county-owned property and arrange with private landowners and homeowner associations for permission to access private properties.
Trappers use a variety of methods to remove iguanas. When safe, they use .22 caliber rat shot or air rifles to shoot iguanas, and place baited live traps in areas frequented by iguanas. In addition, non-baited traps are often set in areas where iguanas have been recently sighted and/or tracked. These traps (referred to as colony traps) are placed in such a manner that iguanas must eventually enter them (Figure 18). Traps are typically located near tortoise burrows, under bushes, or in places where residents specifically report an iguana. The traps are placed in inconspicuous locations at the request of housing associations so they are not tampered with. Evidence of recent iguana activity is used to determine exactly where to set traps. Black spiny-tailed iguana tracks are easily identifiable in sandy areas throughout the island, including the beach dunes and on tortoise burrow aprons. They leave behind footprints on either side of a distinctive “tail drag,” often with visible ridges left behind from the tail’s spiny scales (Figure 19). They also leave distinct scratch marks on tree branches where they perch, especially those of seagrape (Coccoloba uvifera). Traps consist of a rectangular metal cage with a door connected to a flap on the floor that closes the door when stepped on by an iguana. Maraschino cherries make an ideal bait because their bright red color is attractive to iguanas, and they are durable due to added preservatives. Fruits such as watermelon and cantaloupe are also effective baits, but rot quickly and must be replaced frequently.

Credit: Sean L. McKnight, UF/IFAS

Credit: Sean L. McKnight, UF/IFAS
Since 2008, USDA has removed over 15,000 iguanas from the Charlotte County portion of the island, and private trappers have removed many thousands of iguanas from Lee County, as well. These efforts appear to be successful in suppressing numbers of iguanas, but management must be ongoing (Avery et al. 2014). Eradication of iguanas from Gasparilla Island is unlikely, especially as there are areas with iguanas that trappers do not have access to, such as private properties without agreements with iguana trappers. Additionally, there has only been minimal iguana removal at Gasparilla Island State Park due to limited staffing and high visibility from park visitors, with only about 600 animals removed from the park since 2012 and no records of prior removals.
Even though it is highly unlikely that the iguanas can ever be eliminated from the island (remember that it only took three iguanas to populate it to begin with), ongoing removal can help manage the population to reduce the impacts to people and the environment. Iguanas are still a common sight on the island and still cause problems. However, many residents who lived there prior to iguana removals say the difference is obvious, and the iguanas were more numerous before their management than they are today.
What You Can Do
Please support iguana trapping efforts. You can help by working with wildlife managers and allowing iguana trappers access to your property and by sharing this information with your neighbors and encouraging them to do the same. If you see an iguana trap, leave it alone. Managers are aware of the iguana situation on Gasparilla Island, so there is no need to report black spiny-tailed iguana sightings on or near the island. However, residents and visitors to Gasparilla Island State Park are encouraged to report iguana sightings to park staff and support increased iguana management in the park to protect the park’s native plants and animals.
Do not feed iguanas. You can protect your valuable plants with cages or screen enclosures and install sheet metal guards around trees, palms, and dock pilings to prevent iguanas from climbing. Avoid planting species iguanas prefer to eat, such as hibiscus, orchids, bougainvillea, roses, garden vegetables, and some fruit trees. Instead, choose plants that are resistant to iguana damage like milkweeds, oleanders, pentas, crotons, and citrus (if not under quarantine), or other plants that are either too tough or too toxic for iguanas to eat (Kern 2004). You can find more information on dealing with iguanas around your home here.
Be a responsible pet owner. When purchasing a pet reptile, consider the cost of care, caging requirements, how long it will live, how large it will grow, and if it will be safe for your household (Johnson et al. 2011). See a useful guide to buying pet reptiles here. Never release a pet into the wild! It is illegal, and either your pet or native species—or very likely both—will end up suffering the consequences (Johson et al. 2017). You can find more advice and resources for dealing with unwanted exotic pets here.
References
Alford, R. A. 1980. “Population Structure of Gopherus polyphemus in Northern Florida.” Journal of Herpetology 14:177. https://doi.org/10.2307/1563851
Avery, M. L., E. A. Tillman, and K. L. Krysko. 2009. “Gopherus polyphemus (Gopher Tortoise), Ctenosaura similis (Gray's Spiny-Tailed Iguana) Predation.” Herpetological Review 40:435.
Avery, M. L., E. A. Tillman, C. Spurfeld, R. M. Engeman, K. P. Maciejewski, J. D. Brown, and E. A. Fetzer. 2014. “Invasive Black Spiny-Tailed Iguanas (Ctenosaura similis) on Gasparilla Island, Florida, USA.” Integrative Zoology 9:590–597. https://doi.org/10.1111/1749-4877.12085
Bomford, M., F. Kraus, S. C. Barry, and E. Lawrence. 2008. “Predicting Establishment Success for Alien Reptiles and Amphibians: A Role for Climate Matching.” Biological Invasions 11:713–724. https://doi.org/10.1007/s10530-008-9285-3
Catano, C. P., and I. J. Stout. 2015. “Functional Relationships Reveal Keystone Effects of the Gopher Tortoise on Vertebrate Diversity in a Longleaf Pine Savanna.” Biodiversity and Conservation 24:1957–1974. https://doi.org/10.1007/s10531-015-0920-x
Charruau, P., J. Juarez, M. Medina, F. Méndez-de-la-Cruz, and J. Pérez Flores. 2020. “Bacterial Flora of Wild Black (Ctenosaura similis Gray, 1831) and Green (Iguana iguana Linnaeus, 1758) Iguanas from a Mexican Caribbean Atoll.” Herpetology Notes 13:369–376.
Clavero, M., and E. García-Berthou. 2005. “Invasive species are a leading cause of animal extinctions.” Trends in Ecology & Evolution 20:110. https://doi.org/10.1016/j.tree.2005.01.003
Dorcas, M. E., J. D. Willson, R. N. Reed, R. W. Snow, M. R. Rochford, M. A. Miller, W. E. Meshaka, et al. 2012. “Severe Mammal Declines Coincide with Proliferation of Invasive Burmese Pythons in Everglades National Park.” Proceedings of the National Academy of Sciences 109:2418–2422. https://doi.org/10.1073/pnas.1115226109
Douglass, J. F., and J. N. Layne. 1978. “Activity and Thermoregulation of the Gopher Tortoise (Gopherus polyphemus) in Southern Florida.” Herpetologica 34:359–374.
Dove, C. J., R. W. Snow, M. R. Rochford, and F. J. Mazzotti. 2011. “Birds Consumed by the Invasive Burmese Python (Python molurus bivittatus) in Everglades National Park, Florida, USA.” The Wilson Journal of Ornithology 123:126–131. https://doi.org/10.1676/10-092.1
Engeman, R. M., B. Constantin, M. Christie, and P. Hall. 2009. “Ctenosaura similis (Black Spiny-Tailed Iguana). Gopherus polyphemus (Gopher Tortoise). Concurrent Burrow Use.” Herpetological Review 40:84.
Engeman, R., E. Jacobson, M. L. Avery, and W. E. Meshaka. 2011. "The Aggressive Invasion of Exotic Reptiles in Florida with a Focus on Prominent Species: A Review.” Current Zoology 57:14. https://doi.org/10.1093/czoolo/57.5.599
Episcopio-Sturgeon, D. J., and E. F. Pienaar. 2019. "Understanding stakeholders' opinions and preferences for non-native pet trade management in Florida." Human Dimensions of Wildlife 24:46–60. https://doi.org/10.1080/10871209.2019.1537016
Ernst, C. H., and J. E. Lovich. 2009. Turtles of the United States and Canada. Second edition. Johns Hopkins University Press, Baltimore, Maryland, USA.
Fitch, H. S., and J. Hackforth-Jone. 1983. “Ctenosaura similis (garrobo, iguana negra, ctenosaur),” in Costa Rican Natural History, edited by D. H. Janzen, University of Chicago Press, Chicago, Illinois, USA.
Fujisaki, I., K. M. Hart, F. J. Mazzotti, K. G. Rice, S. Snow, and M. Rochford. 2010. “Risk assessment of potential invasiveness of exotic reptiles imported to South Florida.” Biological Invasions 12:2585–2596. https://doi.org/10.1007/s10530-009-9667-1
García Rosales, A., A. Arriaga Noguez, and A. Ramírez-Bautista. 2020. “Natural History of the Black Iguana Ctenosaura similis (Squamata: Iguanidae) in Isla Contoy, Quintana Roo, Mexico.” Acta Biológica Colombiana 25:394–402. https://doi.org/10.15446/abc.v25n3.79707
Godley, J. S. 1989. “Comparison of Gopher Tortoise Populations Relocated onto Reclaimed Phosphate-Mined Sites in Florida,” in Gopher Tortoise Relocation Symposium Proceedings. Nongame Wildlife Program Technical Report 5, edited by J. E. Diemer, D. R. Jackson, J. L. Landers, J. N. Layne, and D. A. Wood, 43–58. Tallahassee, Florida, USA.
Guzy, J. C., B. G. Falk, B. J. Smith, J. D. Wilson, et al. 2023. “Burmese Pythons in Florida: A Synthesis of Biology, Impacts, and Management Tools." NeoBiota 80:1–119. https://doi.org/10.3897/neobiota.80.90439
Hardin, S. 2007. “Managing Non-Native Wildlife in Florida: State Perspective, Policy and Practice.” In Managing Vertebrate Invasive Species, Symposium 14. Edited by G. W. Witmer, W. C. Pitt, and K. A. Fagerstone, 43–52. Fort Collins, Colorado, USA.
Hipes, D., D. R. Jackson, K. NeSmith, D. Printiss, and K. Brandt. 2000. Field Guide to the Rare Animals of Florida. Florida Natural Areas Inventory, Tallahassee.
Iannone III, B. V., S. Carnevale, M. B. Main, J. E. Hill, J. B. McConnell, S. A. Johnson, S. F. Enloe, M. Andreu, E. C. Bell, and J. P. Cuda. 2021. “Invasive Species Terminology: Standardizing for Stakeholder Education.” The Journal of Extension 58:27. https://doi.org/10.34068/joe.58.03.27
Jackson, D., and E. G. Milstrey. 1989. “The Fauna of Gopher Tortoise Burrows,” in Gopher Tortoise Relocation Symposium Proceedings. Florida Game and Fresh Water Fish Commission Nongame Wildlife Program Technical Report 5 edited by J. E. Diemer, D. R. Jackson, J. L. Landers, J. N. Layne, and D. A. Wood, 86–98., Tallahassee, Florida, USA.
Johnson, S. A., M. E. McGarrity, and D. Smith. 2011. “Buyers’ Guide to Pet Reptiles: WEC312/UW357, 8/2011”. EDIS 2011 (8). https://doi.org/10.32473/edis-uw357-2011
Johnson, S. A., M. E. McGarrity, and D. Smith. 2017. “Options for Unwanted Exotic Pets. WEC308/UW353, 6/2017.” EDIS 2017. https://edis.ifas.ufl.edu/publication/UW353. Accessed 29 Sep 2022.
Kern, Jr., W. H. 2004. “Dealing with Iguanas in the South Florida Landscape: ENY-714/IN528.” EDIS 2004 (15). https://doi.org/10.32473/edis-in528-2004
King, W., and T. Krakauer. 1966. “The Exotic Herpetofauna of Southeast Florida.” Quarterly Journal of the Florida Academy of Sciences 29:144–154.
Krysko, K. L., F. W. King, K. M. Enge, and A. T. Reppas. 2003. “Distribution of the Introduced Black Spiny-Tailed Iguana (Ctenosaura similis) on the Southwestern Coast of Florida.” Florida Scientist 66:141–146.
Krysko, K. L., K. W. Larson, D. Diep, E. Abellana, and E. R. McKercher. 2009. “Diet of the Nonindigenous Black Spiny-Tailed Iguana, Ctenosaura similis (Gray 1831)(Sauria: Iguanidae), in Southern Florida.” Florida Scientist 72:48–58.
Krysko, K. L., L. A. Somma, D. C. Smith, C. R. Gillette, D. Cueva, J. A. Wasilewski, K. M. Enge, et al. 2016. “New Verified Nonindigenous Amphibians and Reptiles in Florida, 1976 through 2015, with a Summary of over 152 Years of Introductions.” Reptiles & Amphibians 23:110–143. https://doi.org/10.17161/randa.v23i2.14119
Landers, J. L., W. A. McRae, and J. A. Garner. 1982. “Growth and Maturity of the Gopher Tortoise in Southwestern Georgia: Bulletin of the Florida State Museum.” Biological Science 27:81–110.
Lee, J. C. 2000. A Field Guide to the Amphibians and Reptiles of the Maya World: The Lowlands of Mexico, Northern Guatemala, and Belize. Cornell University Press, Ithaca, New York, USA.
Lockwood, J. L., D. J. Welbourne, C. M. Romagosa, P. Cassey, N. E. Mandrak, A. Strecker, B. Leung, et al. 2019. “When Pets Become Pests: The Role of the Exotic Pet Trade in Producing Invasive Vertebrate Animals.” Frontiers in Ecology and the Environment 17:323–330. https://doi.org/10.1002/fee.2059
McCleery, R. A., A. Sovie, R. N. Reed, M. W. Cunningham, M. E. Hunter, and K. M. Hart. 2015. “Marsh rabbit mortalities tie pythons to the precipitous decline of mammals in the Everglades.” Proceedings of the Royal Society B: Biological Sciences 282:20150120. https://doi.org/10.1098/rspb.2015.0120
Miller, M. A., J. M. Kinsella, R. W. Snow, M. M. Hayes, B. G. Falk, R. N. Reed, F. J. Mazzotti, C. Guyer, and C. M. Romagosa. 2018. “Parasite Spillover: Indirect Effects of Invasive Burmese Pythons.” Ecology and Evolution 8:830–840. https://doi.org/10.1002/ece3.3557
Mushinsky, H. R., and E. D. McCoy. 1994. “Comparison of Gopher Tortoise Populations on Islands and on the Mainland in Florida,” in Biology of North American Tortoises, edited by R. B. Bury, and D. J. Germano, 39–48. United States Fish and Wildlife Service, Washington, D.C., USA.
Mushinsky, H. R., D. S. Wilson, and E. D. McCoy. 1994. “Growth and Sexual Dimorphism of Gopherus polyphemus in Central Florida.” Herpetologica 50:119–128.
Nuñez, L. P. 2016. “Molecular Analyses of Three Non-Indigenous Squamate Species in Florida: Testing Various Hypotheses Regarding Species Introductions.” Thesis, University of Florida, Gainesville, Florida, USA.
Pimentel, D., R. Zuniga, and D. Morrison. 2005. “Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States.” Ecological Economics 52:273–288. https://doi.org/10.1016/j.ecolecon.2004.10.002
Skoloff, B. 2006. “In Boca Grande, Iguanas Run Amok.” Associated Press 14 April 2006. https://www.ocala.com/story/news/2006/04/14/in-boca-grande-iguanas-run-amok/31155739007/. Accessed 29 Sep 2022.
Stemle, L., B. B. Rothermel, and C. A. Searcy. 2022. “GPS Technology Reveals Larger Home Ranges for Immature Gopher Tortoises.” Journal of Herpetology 56 (2): 172–9. https://doi.org/10.1670/20-128
Sylvester, W. R. B., V. Amadi, R. Pinckney, C. N. L. Macpherson, J. S. McKibben, R. Bruhl-Day, R. Johnson, and H. Hariharan. 2014. “Prevalence, Serovars and Antimicrobial Susceptibility of Salmonella spp. from Wild and Domestic Green Iguanas (Iguana iguana) in Grenada, West Indies.” Zoonoses and Public Health 61:436–441. https://doi.org/10.1111/zph.12093
Townsend, J. H., K. L. Krysko, and K. M. Enge. 2003. “The Identity of Spiny-Tailed Iguanas, Ctenosaura, Introduced to Florida, USA (Squamata: Sauria: Iguanidae).” Herpetozoa 16:67–72.
Wilson, D. S., H. R. Mushinsky, and E. D. McCoy. 1994. “Home Range, Activity, and Burrow Use of Juvenile Gopher Tortoises (Gopherus polyphemus) in a Central Florida Population,” in Biology of North American Tortoises, edited by R. B. Bury, and D. J. Germano, 147–160. Fish and Wildlife Research, United States Department of the Interior, National Biological Survey.
Witz, B. W., D. S. Wilson, and M. D. Palmer. 1991. “Distribution of Gopherus polyphemus and Its Vertebrate Symbionts in Three Burrow Categories.” American Midland Naturalist 126:152–158. https://doi.org/10.2307/2426159




